

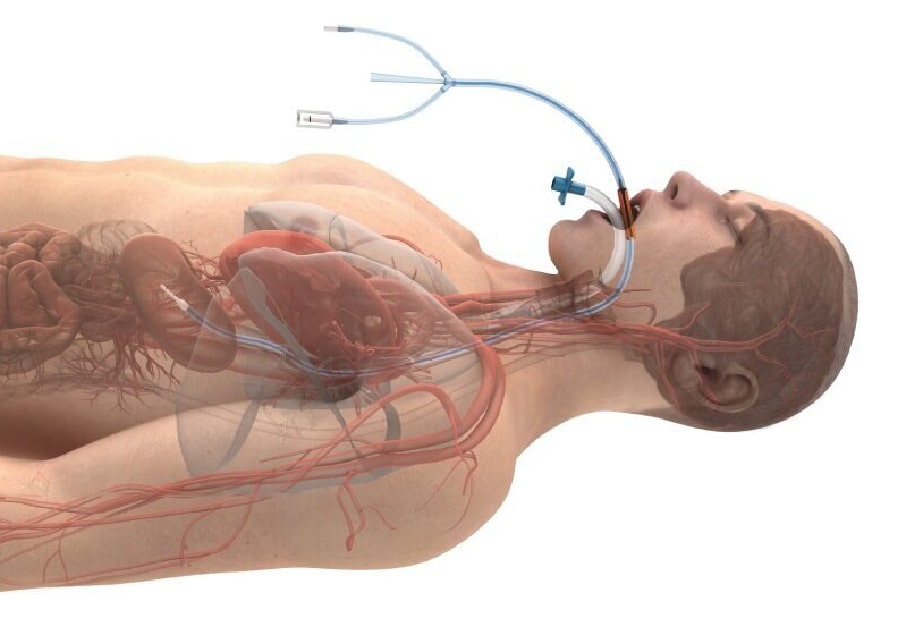
Accurate to the core
The ensoETM® controls temperature through a single-use tube inserted into the esophagus, similar to a standard orogastric tube, and connected to an external heat exchange unit. Positioned at the core, water circulates inside the closed loop system to efficiently cool or warm through the esophagus.
Compatible
The ensoETM device works with existing heat exchangers, so there’s no new capital to purchase. Download product specs for:
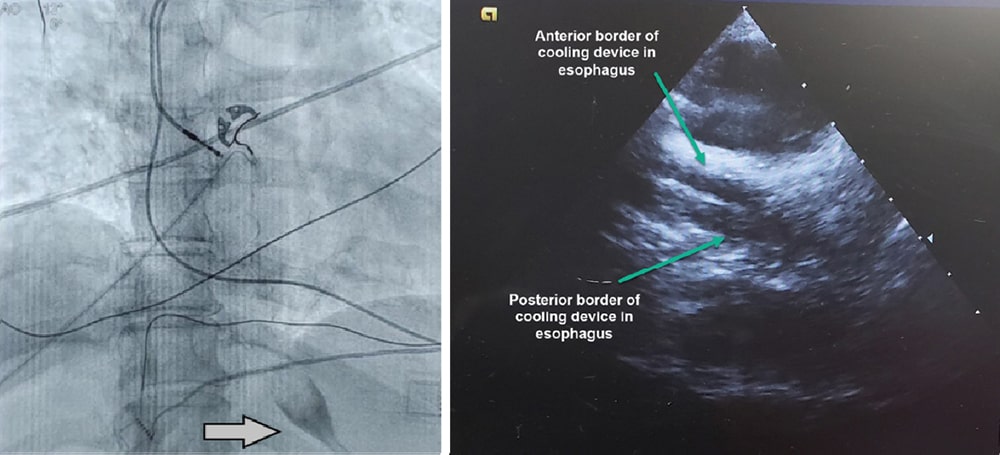
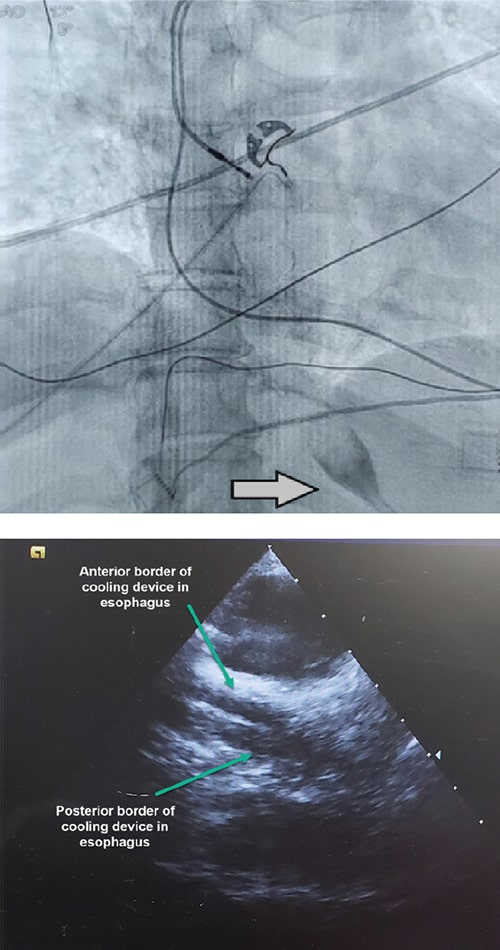
Placing ensoETM
The radiopaque tip of the ensoETM allows you to quickly ensure perfect placement with X-ray or fluoroscopy. Placement may also be confirmed with intracardiac echocardiography (ICE). For more videos on placing ensoETM, use the link below.
 This site is intended for US products only.
This site is intended for US products only.